Fouling in Heat Exchangers
Fouling is the formation of unwanted material deposits on heat transfer surfaces during process heating and cooling.
It occurs in all industries and most heat exchanger designs, with impacts ranging from heat transfer degradation to flow resistance and pressure drops. By forming a kind of insulation on heat-transfer surfaces and reducing heat transfer, fouling undermines heat exchange efficiency.
In this article, we will outline:
- The costs, types, and causes of fouling
- How to detect fouling
- How to clean your heat exchanger
- How to prevent fouling

Costs of fouling
Fouling prevention for heat exchangers is typically focused around the heat exchangers itself, however the fouling and heat exchanger performance can be affected by system characteristics that are present both before and after the heat exchanger. These characteristics can include varying product properties, product handling prior to the heat exchanger and operational performance of other equipment, such as pumps, valves and back pressure.
~ Melissa Fryer, Sanitary Heat Transfer Business Development Manager, Alfa Laval
In short, the costs of fouling are many and varied.
- Production inefficiencies from pressure drops and poor heat transfer
- Maintenance time and equipment for removal of heavy fouling deposits
- Cleaning equipment & chemicals
- Hazardous cleaning solution disposal
- Reduced service life and added energy costs
- Production loss from shutdowns
- Replacement of plugged equipment
- Decreased regeneration efficiency

Types of fouling
The most commonly occurring types of fouling and aging in hygienic processing fall into four main types:
- Incrustation: the accumulation of a crust or coating of processed fluids, minerals, or cleaning agents on the surface of heat exchanger parts.
- Scaling: a type of incrustation caused by calcium carbonate, calcium sulfate, and silicates.
- Sediment: comes from corrosion products, metal oxides, silt, alumina, and diatomic organisms (microalgae) and their excrement.
- Biological growth: Sources of biofouling include bacteria, nematodes, and protozoa.
Causes of fouling in heat exchangers
Several variables contribute to fouling, including water pH, product viscosity, and the roughness of component surfaces, among many others.
Together, the variables can be expressed as a fouling factor that numerically represents resistance to heat transfer — or thermal resistance — in your system.
Key fouling factors
Dairy applications include fats, sugars, and proteins that contribute to fouling. In food and beverage applications, particulate matter can clump and clog, and in pharmaceutical applications, cosmetic and pharmaceutical particles produced during processing can stick and accumulate. To help control fouling, operators pay attention to four key processing factors:
- Fluid velocity
- Fluid temperature
- Fluid chemistry
- Materials of fabrication

Fluid velocity
In most cases, fouling decreases at higher fluid velocities because increasing flow velocity increases the fluid shear stress, which causes more removal of deposits.
For more substantial deposits, increasing the flow velocity beyond a particular point may not decrease fouling significantly. In the case of very strong deposits, increasing flow velocity may not have any effect.

Fluid temperature
Water can produce scaling from minerals such as calcium carbonate (CaCO3). Salts deposit on the heat exchanger surface increases with an increase in temperature.
Similarly, biological growth can occur with an increase in temperature during food processing. Finally, proteins and fats in food products can burn if the temperature difference between the hot product outlet and the hot medial inlet is too significant.

Fluid chemistry
During milk processing, calcium phosphate and whey protein can build up on heat exchanger surfaces. The fouling causes pressure drop increases by reducing the area in which the product can flow. In dairy products generally, proteins, fats, sugars, and minerals can come out of solution and deposit on heat exchanger surfaces and foul channels.
If chemical changes occur within a fluid, the difference can result in a layer of fouling on tube or plate surfaces. For example, suspended salts may harden with an increase in temperature.

Materials of fabrication
To prevent corrosion fouling from layers of thermal-resistant material, select units fabricated with corrosion-resistant stainless steels and alloys. Plates, tubes, and entire heat exchanger units may be fabricated with AISI 304 or AISI 316L stainless steel.
For more corrosive, high-salt products, parts of units may be manufactured with titanium, stainless steel alloys, or Super Alloys™ AL-6XN® and Hastelloy® C-22®.
How to detect fouling in heat exchangers
Fouling detection typically occurs by physical inspection or by monitoring system performance.
Physical inspection includes measuring the fouling's thickness on plates, pipes, or tubes.
- Heat transfer: because of the insulating properties of fouling, heat transfer can fall outside of operating specifications.
- Pressure drop: Pressure drop increases between heat exchanger inlet and outlet may indicate frictional resistance or blocked flow paths caused by fouling deposits.
Monitoring temperature and pressures are the best way to troubleshoot and detect fouling in heat exchangers. Instruments measure and report critical variables that can indicate heat exchanger fouling.

Temperature transmitters
Monitor the temperature of fluids passing through heat exchangers. Temperature drops may indicate loss of heat transfer caused by fouling.

Flow Meters
Indicate decreases that may be caused by build-up of fouling material in pipes and plates.
Heat exchanger cleaning
Cleaning is required to maintain heat exchanger efficiency. Cleaning-In-Place (CIP) equipment circulates cleaning chemicals and rinses to flush interior surfaces of heat exchangers without disassembling them.
Importance of flow rate during cleaning
The proper flow rate ensures the effective mechanical action of fluids during cleaning. Some manufacturers recommend a minimum of 1 ft/sec velocity across heat exchanger plates, but requirements vary by manufacturer.
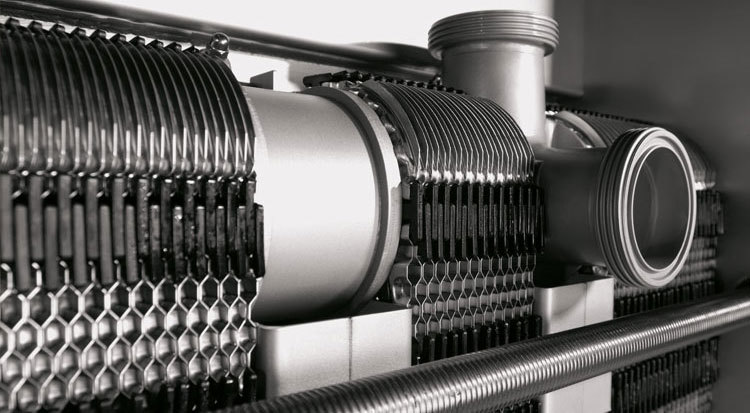
During the cleaning of the product side, the flow rate should always be at least the same as the production's flow rate.
An increased flow rate may be required in some cases—for example, in milk sterilization and the processing of viscous liquids or liquids containing particles.
Cleaning agents to use, by purpose
Incrustation, scaling
Cleaning incrustation or scaling is a process of removing calcium carbonate, calcium sulfate, or silicates from plate surfaces. Cleaning agents must be compatible with both the plate metal and the composition of gaskets.
In titanium and stainless steel plates, never use hydrochloric acid. Also, never use water of more than 300 ppm of chlorine during the preparation of cleaning solutions. In every case where chlorination of non-titanium equipment cannot be avoided, consult your equipment supplier.
Removing sediment
Sediment most commonly consists of metal Oxides, silt, Alumina, and Diatomic organisms and their excrement. Sediment accumulates because heat releases minerals and other particles from fluids during processing cycles, and those settle and deposit on heat transfer surfaces.
You should never use hydrochloric acid with stainless steel or titanium plates because the acid causes general corrosion, pitting, and stress corrosion cracking.
Removing biological growth
When using heat exchangers to increase the temperature of processed foods, biological growth such as bacteria, nematodes, and protozoa can occur. Removing the growth requires the same attention to plate and gasket composition as incrustation.
Manufacturers can provide more detailed information about cleaning and sterilization for specific equipment.
Chemical cleaning basics
CIP operators typically follow four steps in the chemical cleaning process:
- Alkaline clean: removes the build-up of organic materials
- Rinse: generally completed with a high-flow water flusher to remove loose debris and remaining residue from the alkaline step
- Acid cleaning: helps dissolve and soften fouling materials more deeply
- Final rinse
Recommended limits for cleaning solutions
- 5% by volume caustic at a maximum of 70°C
- 0.5% by weight acid solution at a maximum of 70°C

The CIP process for plate and frame heat exchangers
The basic process for CIP is a one-person job that includes
- Connecting a CIP unit to the heat exchanger
- Mixing and heating water and cleaning chemicals
- Alkaline mix: circulating to remove build-up of organic materials
- Rinse: generally completed with a high-flow water flusher to remove loose debris and remaining residue from the alkaline step
- Acid mix: circulating to dissolve and soften fouling materials more deeply
- Final rinse
- Draining the heat exchanger
- Disconnecting the CIP unit

Shell and tube cleaning
Shell-and-tube heat exchangers operate at comparatively low internal fluid velocities so are more susceptible to fouling than plate heat exchangers. To maintain efficient operation and higher transfer coefficients, keep the shell and tube heat transfer surfaces clean.
Cleaning chemicals depend on the same variables for a plate-and-frame heat exchanger, and cleaning compounds must be compatible with the heat exchanger's metallurgy.
In all cleaning processes, operators must use proper protective equipment, such as safety boots, safety gloves, and eye protection to avoid injury.
How to prevent fouling in heat exchangers
The best method to reduce fouling is to keep it from happening in the first place by utilizing a heat exchanger that is properly sized for the application, giving adequate velocities, surface area, and temperature splits.
~ Melissa Fryer, Sanitary Heat Transfer Business Development Manager, Alfa Laval
While fouling is not 100% preventable, several steps can increase the intervals between maintenance. Achieving fouling resistance requires attention to:
- Material surfaces
- Constant flow
- Backflushing
- Port filtration

Material surfaces
Rougher surfaces increase fouling by collecting particles, so select heat exchangers made from 304, 316, or corrosion-resistant plates, pipes, and tubes.
Establish manufacturer-recommended cleaning regimens to prevent excessive build-up of particles.

Constant flow
Maintaining uniform and constant flow of process fluids across heat transfer surfaces generally produces less fouling by preventing particulates from sticking and accumulating.
Particles suspended in process fluids tend to deposit in low-velocity regions, especially where the velocity changes quickly.

Backflushing
Backflushing employs a valve that reverses the flow of fluids through the heat exchanger to force out particulates at the exchanger inlet.

Port filtration
A port filter prevents particles from even entering the unit to reduce the amount of fouling.
Guide to Choosing the Right Heat Exchanger
This guide is designed for processors, production managers, and mechanical engineers to help in the heat exchanger selection process.
Next steps
As you've learned, fouling in heat exchangers can lead to heat transfer degradation, flow resistance, and pressure drops, which can make the proper selection for your process vital. We're here to help!
For replacement parts to keep your current units operating, direct replacement for a worn out or inefficient heat exchanger, or a new unit for a new process, contact CSI today at 417-831-1411.
Our customer service team, engineers, designers, and product specialists provide solutions through a broad range of brands, technologies, and capabilities.
Contributing Author

Melissa Fryer is the Sanitary Heat Transfer Business Development Manager for Alfa Laval’s Food Heat Transfer team. Melissa received her B.S. degree in Chemical Engineering from The State University of New York at Buffalo, and brings over 25 years’ experience in specifying, calculating and troubleshooting heat exchangers in the food, beverage and dairy market. Melissa focuses on engineering solutions to meet customer needs and possesses extensive knowledge of and passion for this industry.
ABOUT CSI
Central States Industrial Equipment (CSI) is a leader in distribution of hygienic pipe, valves, fittings, pumps, heat exchangers, and MRO supplies for hygienic industrial processors, with four distribution facilities across the U.S. CSI also provides detail design and execution for hygienic process systems in the food, dairy, beverage, pharmaceutical, biotechnology, and personal care industries. Specializing in process piping, system start-ups, and cleaning systems, CSI leverages technology, intellectual property, and industry expertise to deliver solutions to processing problems. More information can be found at www.csidesigns.com.

