Advantages of Clean-in-place (CIP) Systems

Making food and pharmaceuticals safe is a primary goal for all processing plants, and clean in place is an important tool for reaching that goal. CIP systems designed especially for the food, dairy, beverage, and pharmaceutical processing industries deliver cleaning processes to avoid risks to product quality and purity.
CIP equipment helps control, monitor, and document the cleaning methods essential to hygienic processing. As clean-in-place technologies have become more efficient and cost effective, cleaning process systems in-place not only helps ensure product safety but can also add efficiencies to cleaning processes.
What is CIP?
Cleaning in place (CIP) includes several activities that combine to properly clean all or part of a process system without having to take the system apart. CIP systems pump cleaning, rinsing, and sanitizing solutions through the same piping path as the product to eliminate product soil from internal surfaces.
CIP systems vary widely in configuration, capacity, quality, and level of automation. They also vary by industry. Differences in product characteristics and regulatory considerations between various industries impact the design of a CIP system.
Food, Dairy, and Beverage
The number of CIP applications for food, dairy, and beverage process systems has grown significantly in recent years. In most food, dairy and beverage systems, the surface finish for product contact areas tends to be the standard sanitary surface finish of 32Ra minimum. This is rougher than the finishes required in the pharmaceutical world, but it is smooth enough to allow most product residues to release easily.
Typical CIP cycles
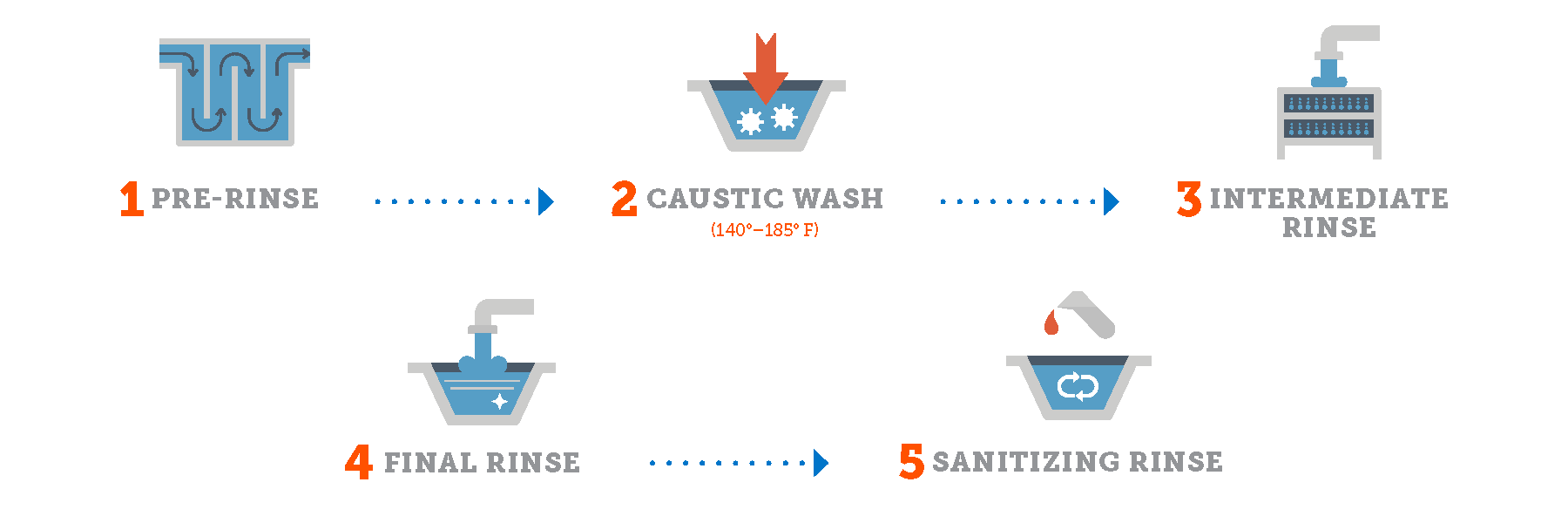
Beverage CIP applications require less time and energy and fewer chemicals than some applications because the product is less viscous and doesn’t contain proteins or fats. The acidity of many beverages also naturally inhibits the growth of microorganisms, which may mean cleaning less frequently.
Milk and dairy products are particularly vulnerable to spoilage and the rapid growth of bacteria.
Clean-in-place systems in dairies must be very specific regarding temperatures, cycle times and chemical concentrations to effectively prevent contamination from harmful microorganisms such as salmonella, listeria and E. coli.
Additional acid washes may also be needed to eliminate milk scale buildup. Pasteurizers and other equipment containing heating surfaces (known as “hot components”) may require separate cleaning programs from the non-heated components of the system such as tanks and piping.
Breweries
Most modern breweries are highly automated closed systems, so they lend themselves well to cleaning in place. Tanks and vessels used in brewing sometimes have a large capacity and may require multiple high-capacity spray devices to be properly cleaned. A phosphoric acid wash may be required in some cases to remove beerstone buildup.
Pharmaceutical clean-in-place
The highest level of CIP technology and state-of-the-art controls are usually found in pharmaceutical CIP systems.
The exacting standards of the industry demand a level of
- Hygiene
- Documentation
- Automation
For most pharmaceutical processing applications, a minimum roughness average of 20Ra for product contact surfaces is dictated by ASME BPE standards, so residual product doesn’t adhere well to surfaces, making it easier to remove.
Process line sizes in pharmaceutical processing tend to be smaller and the product is often less viscous than many food products, so high flow volumes and powerful spray devices may not be necessary. The design of pharmaceutical process piping should minimize deadlegs, be sloped properly and should be free of undrainable low points.
Pumps, valves, and fittings used in pharmaceutical processing generally have the highest level of cleanability designed into them, which makes them easier to clean and less likely to have hidden, hard-to-clean areas. A sterilization cycle is commonly included in many pharmaceutical CIP programs.

Keys to Proper Design
Depending on the system and the product being cleaned, a CIP system may be as simple as a self-contained skid to clean one small circuit or as complex as a large sophisticated system equipped to service multiple circuits or kitchens simultaneously.
Before purchasing or upgrading a clean-in-place system, assess system needs and existing equipment to ensure the design plan optimizes cleaning and minimizes the use of resources
- CIP works with equipment and piping designed for CIP. Not all equipment is designed for CIP, such as tanks with lift-off lids.
- Whenever possible, run piping-circuit cleaning cycles separately from tank-cleaning cycles.
- Extremely long piping circuits may have to be cleaned in shorter circuit sections.
- Don’t try to clean large-diameter piping and small diameter piping at the same time.
- Clean tanks and vessels individually whenever possible.
- Ensure that every component in the line receives a turbulent flow rate.

Advantages of CIP systems
Because CIP systems are designed to integrate with processing equipment, they provide a number of advantages to processing operations.
1. Increased Product Safety
- CIP minimizes mistakes by automating cleaning to reduce the chances of human error that can contribute to an unsafe product.
- CIP decreases contamination risks with monitoring sensors.
2. Increased Employee Safety
- CIP reduces employee chemical exposure by containing cleaning solutions within the system.
- No Vessel Entry – Employees don’t have to enter tanks to clean them.
The Bottom Line
The objective for most process environments is to maximize quality production time and minimize other activities and costs. The bigger and more difficult the cleaning job and the more frequent the cleaning cycle, the more cost effective CIP can be.
Some of the main economic benefits of installing a CIP system
- More Production Time: Less production time lost to cleaning, more time spent making product.
- Product Quality: Reliable and repeatable cleaning leads to sustainable product quality and consistency, fewer product recalls, and higher brand confidence.
- Employee Efficiency: More labor time spent on productive, profitable activities.
- Utility Savings: Water and energy usage reduced through repeatable cycle control.
- Lower Water Treatment Costs: Less effluent down the drain.
Regulatory advantages
As the concern for public safety of food and pharmaceutical products increases, cleaning in place helps maintain required levels of hygiene by meeting regulatory standards.
Food Safety Modernization Act
Signed into law in 2011, the FSMA granted the Food and Drug Administration (FDA) new authority to regulate how food is grown and processed. A major emphasis of the FSMA is the focus on prevention of practices that can lead to foodborne illnesses. Cleaning in place is a valuable tool when it comes to food safety; therefore, a quality CIP system should provide all of the necessary functionality needed to maintain the standards set forth in the FSMA.
CFR21
The Code of Federal Regulations (CFR) is a set of rules published by the U.S. government. Title 21 of the CFR (CFR21) is used by the Food and Drug Administration to establish requirements for the manufacture of food industry products and pharmaceuticals, including how equipment should be cleaned and maintained. A top quality, well-designed CIP system should adhere to these guidelines and should comply with CFR21.
S88
In recent years a set of standards known officially as ANSI/ISA-88 was developed to address batch process control procedures and provide standard organization for how systems communicate and work together. A CIP system that complies with S88 would have an advantage when it comes to integrating into a process system.
CIP equipment controls, monitors, and documents cleaning methods that are essential to sanitary processing. Industries invest in CIP systems to reduce downtime while increasing employee safety and product quality. According to Transparency Market research, CIP is on an annual growth rate of over 15%, suggesting that, with industry penetration increasing each year, processors who don’t use CIP may be at a competitive disadvantage.
Next Steps
Understanding the advantages of a CIP Cycle helps to ensure product integrity.
However, purchasing and installing a CIP system for your processing facility is a considerable undertaking, requiring analysis, planning and above all, partners.
That’s where we come in.
CSI has the ability to engineer, design, and fabricate a custom Clean-in-place System to meet your exact hygienic processing needs.
With CSI's state-of-the-art, climate-controlled fabrication shop, the quality of equipment leaving our facility is second to none. We offer in-house, Level II inspection in accordance with the latest ASNT recommended Practice No. SNT-TC-1A, so you can be certain your equipment meets industry standards.
To speak with a CIP expert, request a quote below or call 800.654.5635.
Clean-in-place Buying Guide
This Buying Guide for Clean-in-Place Solutions is a comprehensive resource for anyone who designs, owns, or operates processing systems and wants information about all aspects of CIP Systems.
ABOUT CSI
Central States Industrial Equipment (CSI) is a leader in distribution of hygienic pipe, valves, fittings, pumps, heat exchangers, and MRO supplies for hygienic industrial processors, with four distribution facilities across the U.S. CSI also provides detail design and execution for hygienic process systems in the food, dairy, beverage, pharmaceutical, biotechnology, and personal care industries. Specializing in process piping, system start-ups, and cleaning systems, CSI leverages technology, intellectual property, and industry expertise to deliver solutions to processing problems. More information can be found at www.csidesigns.com.

